Why 2-Phenanthrol Stands Out Among Phenanthrol Isomers
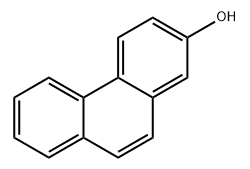
Phenanthrol is a group of hydroxylated derivatives of phenanthrene, including 1-, 2-, and 9-Phenanthrol. Among them, 2-Phenanthrol is particularly noteworthy due to the hydroxyl group attached at the second carbon position. This seemingly minor structural variation has profound implications for both chemical reactivity and biological activity. The hydroxyl group at this position facilitates hydrogen bonding, affects electronic distribution across the aromatic rings, and creates specific steric interactions that other isomers do not possess. These differences make 2-Phenanthrol more selective in reactions such as oxidation, electrophilic substitution, and photochemical transformations. Its chemical stability and predictable reactivity make it a preferred choice for researchers needing precise and reproducible results, particularly in pharmacological and material science experiments.
Key Properties Buyers Should Know
High-purity 2-Phenanthrol is typically supplied as a crystalline solid or powder with purity levels exceeding 98%. It is soluble in polar organic solvents like ethanol, DMSO, and acetone, which is crucial for experimental consistency. Melting point ranges around 115–118°C, indicating its crystalline nature and ease of handling. Buyers should also note its thermal and chemical stability; it remains stable under standard lab conditions but can degrade if exposed to moisture, strong oxidants, or prolonged light exposure. Quality assurance from the supplier is critical—look for a Certificate of Analysis (CoA) confirming purity, water content, and absence of by-products. Understanding these properties ensures that laboratories and industrial users can rely on 2-Phenanthrol for reproducible and accurate results.
Common Uses of 2-Phenanthrol
2-Phenanthrol’s most significant role is in biomedical research as a selective TRPM4 ion channel inhibitor. By modulating ion flow in cardiac and neuronal tissues, it enables scientists to study cardiac arrhythmias, neuronal excitability, and related pathophysiological mechanisms. Beyond pharmacology, it serves as a versatile intermediate in organic synthesis. Chemists use it to create functional dyes, polymers, and other aromatic compounds, taking advantage of its hydroxyl group for further chemical modifications. In addition, its unique electronic properties make it useful for photochemical studies and material science applications, including developing light-sensitive materials and specialized organic semiconductors. This wide range of uses highlights why 2-Phenanthrol is often prioritized over other phenanthrol isomers.
Choosing the Right Phenanthrol Isomer for Your Needs
Selecting the appropriate phenanthrol isomer depends heavily on the intended application. While 1-Phenanthrol is sometimes preferred for general synthetic chemistry and 9-Phenanthrol for photochemical investigations, 2-Phenanthrol’s specific biological activity makes it indispensable in ion-channel research. Buyers should consider several factors: solubility in the target solvent system, chemical reactivity for downstream reactions, stability under storage conditions, and compatibility with other experimental reagents. For pharmaceutical or biotech laboratories, prioritizing 2-Phenanthrol can save time and improve data consistency, whereas material science applications may require additional evaluation of physical properties such as crystallinity and thermal behavior. In practice, evaluating supplier data, CoAs, and experimental requirements ensures informed selection.
Safety and Handling Tips
While 2-Phenanthrol is not classified as highly hazardous, safe handling remains essential. Laboratory personnel should use gloves, goggles, and work in a well-ventilated environment or fume hood. Avoid inhalation, skin contact, and ingestion. Storage should be in airtight containers, away from moisture, strong oxidizers, or direct sunlight. Proper labeling and adherence to local chemical disposal regulations are critical to prevent contamination or environmental hazards. For industrial users, understanding Material Safety Data Sheets (MSDS) and incorporating standard operating procedures (SOPs) ensures both safety and compound integrity, reducing the risk of accidental degradation or exposure.
Where to Buy High-Quality 2-Phenanthrol
Sourcing 2-Phenanthrol from reputable suppliers is essential to maintain experimental reliability and reproducibility. Look for distributors who provide detailed Certificates of Analysis (CoA), including purity, water content, and batch traceability. Suppliers with technical support can offer guidance on handling, storage, and recommended applications, which is especially valuable for new users or large-scale industrial purchases. Competitive pricing is important, but quality and reliability should take precedence. For researchers or companies aiming for consistent results, partnering with trusted suppliers ensures high-quality 2-Phenanthrol with minimal risk of impurities or variability.
2-Phenanthrol is a unique phenanthrol isomer whose distinct hydroxyl positioning provides unmatched advantages in chemical reactivity, biological activity, and versatility across research and industrial applications. Understanding its properties, uses, handling requirements, and sourcing considerations empowers buyers and researchers to fully leverage its potential.



